Spondyloarthritis (SpA)*:
An inflammatory pathway different from RA
*Specifically, psoriatic arthritis (PsA) and ankylosing spondylitis (AS).
Recent investigations have revealed distinctions in the T cell subtypes associated with the pathogenesis of SpA and RA1
The presence of serum rheumatoid factor (RF) and spontaneous RF-secreting B cells is a common feature in most patients with RA.2,3
In contrast, most patients with PsA and AS are RF-negative. Many AS patients in particular are positive for HLA-B27, which is thought to facilitate the role of T cells in the pathogenesis of SpA.4,5
T cells contribute to both the inflammatory and osteoproliferative processes characteristic of SpA. IL-17A-expressing T cell levels are elevated in the bone marrow and synovial fluid of joints in patients with PsA and AS.5-8 Studies have shown blood levels of Th-17 and IL-17+/CD- cells are higher in patients with PsA and AS than in patients with RA.1,7
With SpA, an initial, as yet unknown, trigger activates cells to express cytokines, promoting chronic inflammation and bone erosion and formation.9-11
The pathophysiology of SpA is complex and involves the interplay between multiple cell types and cytokines, including dendritic cells, T cells, TNF-α, IL-6, IL-23, IL-17A, and IL-22.8-10

Take a closer look at the reality of SpA1,6,10-22
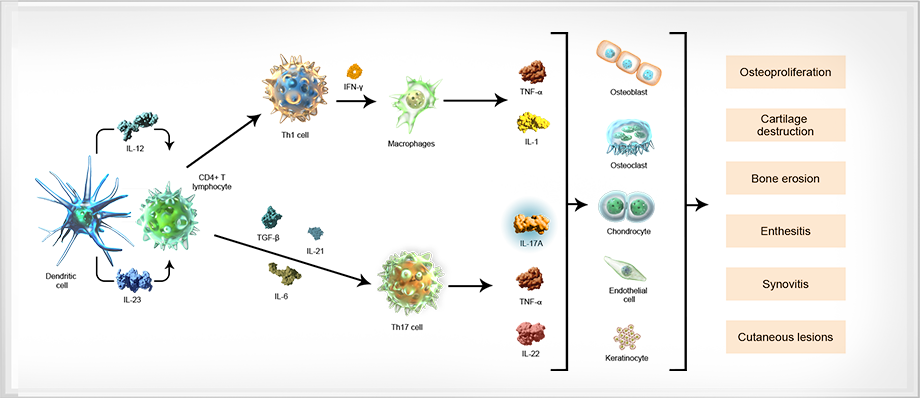
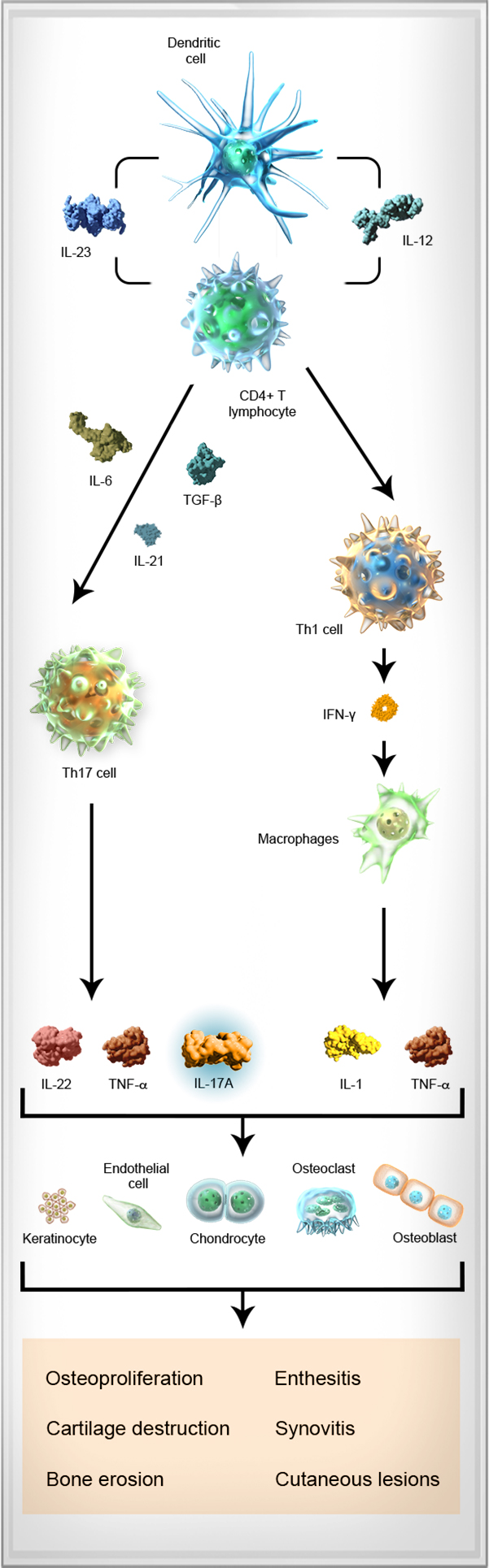

Dendritic cell
Dendritic cell Upon activation (from injury or infection), dendritic cells produce IL-23, a cytokine that mediates the differentiation of naive T cells into Th1, Th2, or Th17 cells.12-14
IL-23
IL-23 IL-23, a cytokine produced by dendritic cells, contributes to the differentiation of naive CD4+ T cells into Th17 cells, and is associated with pathologic changes to the bone and cartilage.10,12,15
CD4+ T lymphocyte
CD4+ T lymphocyte The T cell pathway has been implicated in the pathogenesis of SpA. CD4+ T cells can differentiate into Th1, Th2, and Th17 cells, initiating an inflammatory cascade.6,7,12,16,17
Th17 cell
Th17 cell Th17 cells are derived from CD4+ T cells that have been activated through antigen stimulation and through the action of specific dendritic cell-derived cytokines, such as IL-23, transforming growth factor-β, and IL-6. In turn, Th17 cells secrete several effector cytokines, including IL-17A, TNF-α, and IL-22.12,18
IL-17A
IL-17A IL-17A is produced by Th17 cells, and by certain cells of the innate immune system, such as neutrophils and mast cells.8,12
IL-17A has an inflammatory effect on a number of cells including macrophages, epithelial cells, osteoblasts involved in bone erosion, and chondrocytes, which damage cartilage.19
Higher levels of IL-17A and IL-17A-producing cells were found in the joint synovial fluid of patients with PsA and AS than in patients with RA or OA.1,16,17,20
TNF-α
TNF-α Various cells—including T cells, macrophages, mast cells, and keratinocytes—produce TNF-α, leading to cytokine production and immune cell recruitment.21
IL-22
IL-22 IL-22 is a cytokine also produced by Th17 cells. IL-22 has been implicated in helping to drive inflammation and bone formation in SpA.10,11
Osteoblast
Osteoblast Bone loss and joint destruction that are characteristic of SpA diseases are mediated through osteoblast stimulation of osteoclasts.19,22
Osteoclast
Osteoclast T-cell mediated secretion of inflammatory cytokines supports the activation of osteoclasts that can lead to bone loss.18
Chondrocyte
Chondrocyte Inflammatory activity disrupts matrix production in chondrocytes and leads to eventual cartilage damage.5,19,23
Keratinocyte
Keratinocyte Chronic disruptions in the inflammatory signaling responsible for keratinocyte production are thought to underlie skin and joint changes characteristic of psoriatic disease.21
IL=interleukin; OA=osteoarthritis; RA=rheumatoid arthritis; Th=T helper cells; TNF=tumor necrosis factor.
References: 1. Menon B, Gullick NJ, Walter GJ, et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 2014;66(5):1272-1281. 2. Hoffmann M, Lundberg K, Steiner G. Autoantibodies in rheumatoid arthritis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. 6th ed. Philadelphia, PA: Elsevier; 2015:51-82. 3. Nakken B, Munthe LA, Konttinen YT, et al. B-cells and their targeting in rheumatoid arthritis—current concepts and future perspectives. Autoimmun Rev. 2011;11(1):28-34. 4. Rudwaleit M. Classification and epidemiology of spondyloarthritis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. 6th ed. Philadelphia, PA: Elsevier; 2015:83-90. 5. Baeten D. Etiology, pathogenesis, and pathophysiology of ankylosing spondylitis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, eds. Rheumatology. 6th ed. Philadelphia, PA: Elsevier; 2015:102-113. 6. Appel H, Kuhne M, Spiekermann S, et al. Immunohistologic analysis of zygapophyseal joints in patients with ankylosing spondylitis. Arthritis Rheum. 2006;54(9):2845-2851. 7. Jandus C, Bioley G, Rivals J-P, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58(8):2307-2317. 8. Smith JA, Colbert RA. Review: the interleukin-23/interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol. 2014:66(2):231-241. 9. Lories RJ, McInnes IB. Primed for inflammation: enthesis-resident T cells. Nat Med. 2012;18(7):1018-1019. 10. Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8-entheseal resident T cells. Nat Med. 2012;18(7):1069-1076. 11. Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2013:44(2):183-193. 12. Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116(5):1218-1222. 13. Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467-476. 14. Happel KI, Zheng M, Young E, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170(9):4432-4436. 15. Tsui FW, Tsui HW, Akram A, Haroon N, Inman RD. The genetic basis of ankylosing spondylitis: new insights into disease pathogenesis. Appl Clin Genet. 2014;7:105-115. 16. Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL-17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem. 2012;359(1-2):419-429. 17. Appel H, Maier R, Wu P, et al. Analysis of IL-17(+) cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res Ther. 2011;13(3):R95. 18. Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888-898. 19. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763-776. 20. Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60(6):1647-1656. 21. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496-509. 22. Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345-1352. 23. Lories RJ, Luyten FP, de Vlam K. Progress in spondylarthritis: mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther. 2009;11(2):221.


